Center for Bioethics and Medical Humanities (CBMH FK-KMK UGM) held Raboan Discussion Forum on Wednesday (21/12). CBMH FK-KMK UGM invited Dr. Sampurno, MBA, Apt, ex-head of Badan Pengawas Obat dan Makanan Republic Indonesia (2001-2006). Dr. Sampurno presented topic Drug and Food Control. The discussion was lead by Prof. Dr. Sismindari, SU, Apt., Professor at Faculty of Pharmacy UGM.
Drug and food control is crucial because it involves the safety of millions of human lives and the value of large commodity circulation. The monitoring system must be carried out in a systematic, strict, and comprehensive manner based on an efficient system. The purpose of the drug and food control system is to protect people’s health and safety from products that do not meet the requirements and increase national industry excellence.
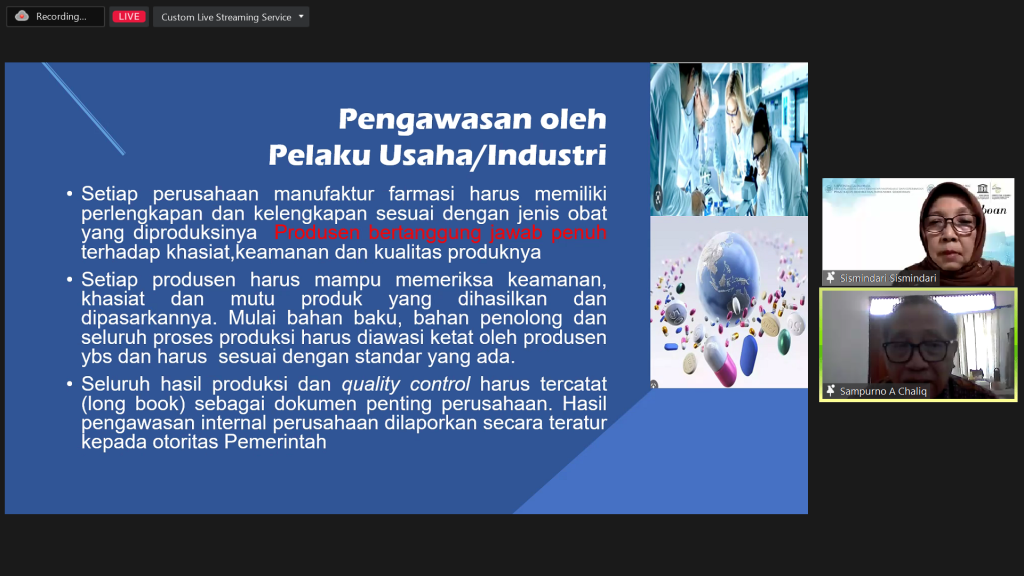
Business/industry actors must be fully responsible for the quality of their products and be able to check the safety, efficacy, and quality of the products they produce and market. All production and quality control results must be recorded as important company documents. Products that must be controlled and supervised are all medicinal products, traditional medicines, food, cosmetics, and medical devices circulating in the country as well as those exported.
Recommendations for drug and food control include the development of a system-based BPOM equipped with up-to-date and sophisticated IT and AI, the development of BPOM institutions throughout Indonesia as a unified monitoring/laboratory network with an effective line of command, the development of BPOM as a learning organization with human capital as the main actors, BPOM’s ability as an initiator of cross-sectoral collaboration and synergy, and the development of leadership with a strategic vision that is broad-minded and able to mobilize public participation and awareness.
Watch full video here
Watch full video here